Reich/Chem 647
Named Effects and Rules in Organic Chemistry
Markovnikov's Rule. In the ionic addition of H-X (or, in general, Y-X), to double bonds, the least electronegative atom (H or Y) adds to the carbon with the most hydrogens on it. The modern version considers these effects in terms of the stability of 1°, 2° and 3° carbonium ions.
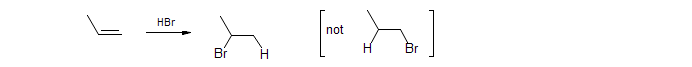
Hammond's Postulate. The structure of transition states resemble either the starting material or product, depending on which is closer in energy. Thus for exothermic reactions the transition state resembles the starting material, for endothermic reactions it resembles the product.
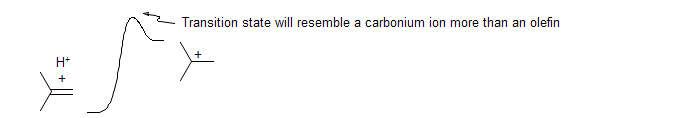
Curtin-Hammett Principle. When two conformations, tautomers, or isomers of a starting material are in rapid equilibrium (compared to rate of a reaction), then the product ratio is independent of the ratio of the starting species. Conversely, the product ratio gives no information about which conformation, tautomer or isomer was present in the starting material. In other words, just because you can see it doesn't mean that it is the reactive species.

Thorpe-Ingold Effect (also known as gem-dimethyl effect). The presence of a quaternary carbon (e.g., a gem-dimethyl group) in a chain increases rates and equilibrium constants of cyclization reactions.
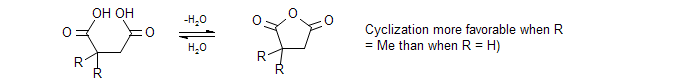
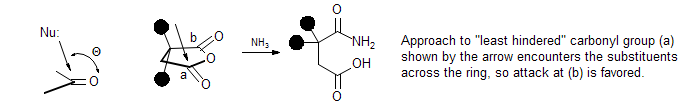
Felkin-Anh Rule for Asymmetric Induction. A mnemonic for predicting the sense of asymmetric induction in addition of nucleophiles to carbonyl groups in compounds having an a-asymmetric center. The model is based on the most favorable trajectory of approach (Burgi-Dunitz trajectory), and emphasizes steric interactions between the nucleophile and substrate. The Cram model predicts the same major product, but is based on a different set of assumptions (also important are the Cram-chelation, Cornforth and Cieplak models).

Burgi-Dunitz Trajectory. Nucleophiles approach C=O, C=N and C=C double bonds at roughly the tetrahedral angle Q (rather than perpendicular to the plane of the carbonyl group along the p p-orbital axis). This provides an explanation for the curious effect that in 2,2-dimethylsuccinic anhydride it is the more sterically hindered carbonyl group that is preferentially attcked by nucleophiles.
Baldwin Rules for Ring closure. When small (6-membered and smaller) rings are formed by intramolecular addition of nucleophiles or radicals to double bonds, then exocyclic addition is preferred.
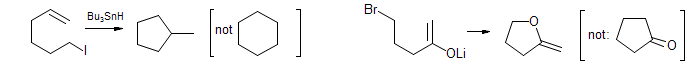
Zimmerman-Traxler Transition State for Aldol Condensation. The chair-cyclohexane-like model for predicting the stereochemical outcome of aldol condensations with stereochemically defined enolates.

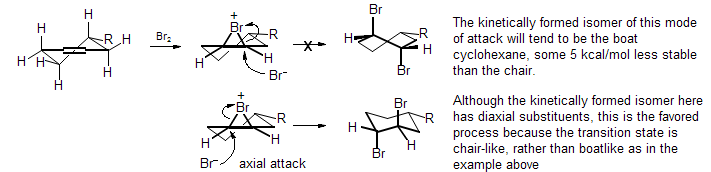
Platt-Fürstner Rule. Cylohexene epoxides undergo ring opening with nucleophiles to give diaxial products.

A corolary is that additions of electrophiles like bromine to cyclohexene double bonds, which go through related bromonium ions, give the diaxial product, even though this is usually the less stable one. These effects fundamentally arise from the inherent preference of cyclohexanes for the chair over the twist-boat conformation.
Hückel Rule. Cyclic conjugated systems show unusual stability (aromaticity) when there are 4n + 2 p electrons in the p orbitals. Thus benzene, cyclopentadienide anion and tropylium cation (6p electrons) show unusual stability, whereas cyclobutadiene and cyclopentadienyl cation (4p electrons) are unusually unstable (antiaromatic).
The Woodward-Hoffmann Rules apply the 4n + 2 electron count to pericyclic transition states. The Dewar-Zimmerman Rules provide a generalized way of treating pericyclic transition states, extending the "aromatic transition state" concept to systems of 4n p electrons with a Moebius cyclic overlap.

Some others to know about: Stork-Eschenmoser Hypothesis, Cornforth, Cram, and Cram-Chelation rules for asymmetric induction.