 |
Molecules in 3D | 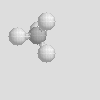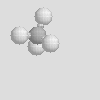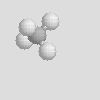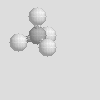 |
 |
Molecules in 3D |  |
Atoms and molecules are so small that we are unable to see them with our naked eyes. To view the three-dimensional structures of molecules we use models. Molecular models generated by computer programs or physical models rely on accurate data derived from experiments on bond lengths, bond angles and atomic radii. To distinguish atoms colors have been assigned. (Take a quick look at the colors. Keep the web page open for future reference.)
Different molecular models emphasize certain features of three dimensional arrangement. For example, the ball-and-stick model helps visualize accurate bond angles. Figure A below shows the ball-and-stick model of the hydrogen molecule. The hydrogen atoms, shown in white, are connected to each other by a short piece of plastic which represents the bond between the two atoms. Figure B shows the same ball-and-stick model for water. As you can see the oxygen atom in the center is connected to two hydrogen atoms. The angle between the two hydrogen atoms is accurate.
 |
 |
Figure A | Figure B |
Identify each of the following diatomic molecules. Write their names and chemical formulas on your data sheet.
Atoms arrange themselves in three-dimensional aggregates with specific molecular shapes. Factors which influence the shape of a molecule are: the number of bonds, non-bonding electrons, atomic radii, bond length among others. The first two factors are the result of electron-electron repulsion. Bonds are made up of two electrons. If a molecule has 3 atoms it is likely that the bonds between the atoms be as far apart as possible, thus reducing repulsion.
|
|
There are some other molecular shapes shown below. On your data sheet write the chemical formula, the name and the molecular shape of each.
| Linear | Bent | Trigonal Planar |
| Trigonal Pyramidal | Tetrahedral |
On your data sheet write the chemical formulas, names and molecular shapes of the molecules listed below.
Compounds made solely of carbon and hydrogen in a chain with all single bonds are called alkanes. Below are the three-dimensional shapes of the first eight alkanes.
There are some other molecules which we will encounter in the course. It is a good idea to get acquainted with their shapes. On your data sheet draw their chemical structures (do not forget double bonds) along with their names.
| Benzene | Ethene or Ethylene |
| Methylamine | Acetic acid or Ethanoic acid |