Polyatomic ions stacked in an ionic crystal lattice can be represented by one "ball" just as ions of a single atoms can. For instance, the carbonate ions, CO32-, is a flat trigonal ion held together by covalent bonds between the C and O atoms.  Calcite, CaCO3, is an ionic compound made up of Ca2+ and CO32- ions. The lattice is very much like the rock salt lattice with Ca2+ replacing Na+ and CO32- replacing Cl -.
|

| Last Page | |
| Menu | Index |
| Time Line | exit |
|
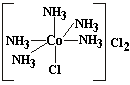
Coordination theory postulates the existence of two types of valence. In these structures, some groups are bound to the metal forming a radical and some are loosely bound to the radical by electrostatic forces (i.e. as ions )
The ionic and loosely bound chlorines in the above structure can be removed by precipitation by silver. The chlorine attached to the metal cannot. In modern day terms, the metal, with its attached groups, is similar to the polyatomic ions we studied earlier in the course. The number of groups attached directly to the metal is the coordination number of the metal.
|
 |
||
|
Alfred Werner |