Jørgensen regards a chain of four ammonia groups to be particularly stable. When we count isomers, we should not break up four-ammonia chains.
|

Given the following structures, which of the theories would predict that Co(NH3)4Cl3 would have isomers?
| Werner | 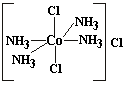 |
|
| Jørgensen | 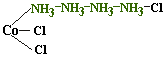 |
|
| Click the mouse on the answer button you choose. When you have chosen your answer, click on the "Done" button. |   |
|
 As
you can see, my theory predicts two isomers for this compound whereas
Jørgensen's does not. This might serve as a test
of our theories. A metal ammine with two Cl groups should not have
isomers according to Jørgensen's theory. According to my
theory, it can.
As
you can see, my theory predicts two isomers for this compound whereas
Jørgensen's does not. This might serve as a test
of our theories. A metal ammine with two Cl groups should not have
isomers according to Jørgensen's theory. According to my
theory, it can. As
you can see, Werner's theory predicts two isomers for this compound
whereas mine does not. This might serve as a test of our theories.
A metal ammine with two Cl groups should not have isomers according
to my theory. According to Werner, it can.
As
you can see, Werner's theory predicts two isomers for this compound
whereas mine does not. This might serve as a test of our theories.
A metal ammine with two Cl groups should not have isomers according
to my theory. According to Werner, it can.