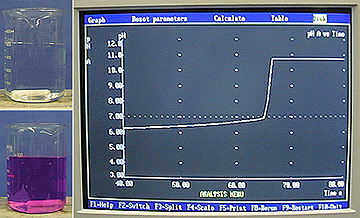

Description: A mixture containing a 10:1 mole ratio of HSO3- : SO32- is prepared. It has a pH of approx. 6. When formaldehyde is added to the solution, the bisulfite is consumed, but not the sulfite. The pH changes as the ratio of bisulfite to sulfite changes. The change in pH is monitored by phenolphthalien indicator and by graphing the pH on a computer
Rating: ![]()
![]()
![]()
Source: Daniel C. Harris, Quanitative Chemical Analysis
Year or vol: 1999 page: 228
Keywords: , , ,