Electron Pair and Molecular Geometry
| Home |
| Table of Contents |
| Gateway Page |
| In this module: |
| Introduction |
| Definitions |
| Electron Pair Geometry |
| Molecular Geometry |
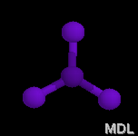
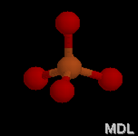
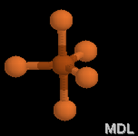
Electron Pair Geometry
|
Now, count the number of electron domains around the central atom. Then, using the following chart, identify the electron-pair geometry shape and name.
Example:
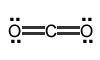
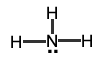
Your turn: Identify the electron-pair geometry of the following molecules: |
 |
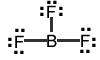 |
 |
Molecular Geometry |