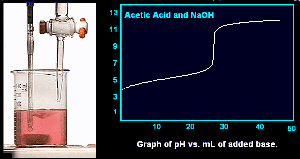
Titration of a weak acid with a strong base.
As a solution of NaOH is added to the a solution of acetic acid. Note the pH at the start is about 3.5.
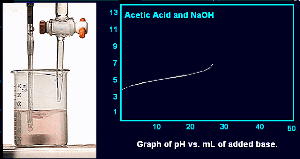
The pH has reached 7 but all of the acid has not been neutralized.
The phenolphthalein indicator has changed from colorless to red indicating that the solution is basic.
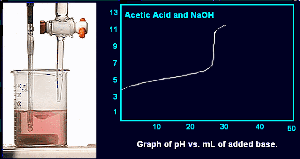
Here is the complete titration curve for acetic acid and sodium hydroxide.