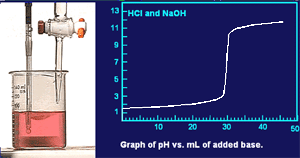
Titration of a strong acid with a strong base.
As a solution of NaOH is added to the HCl a plot of pH vs. volume of added base is made.
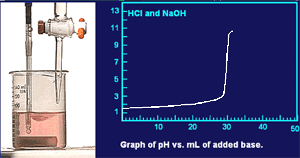
About 30 mL of base have been added. Notice the rapid change in pH.
The phenolphthalein indicator has change from colorless to red indicating that the solution is basic.
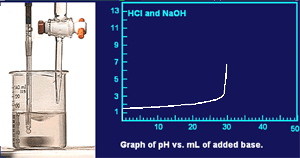
Notice that the pH is not changing rapidly at this point in the titration.