Review Questions Chapter 12
1 Which of the following statements are true of an acid?
1. Its aqueous solution contains more hydroxide than hydrogen ions.
2. Its aqueous solution turns litmus blue.
3. It is a proton acceptor.
4. It is more apt to contain a metal than a nonmetal.
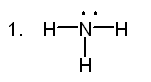
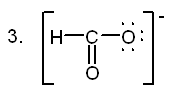
2. Which of the following can act as Brønsted-Lowry bases?

![]()

![]()
3. Which of the following formulas has been given an incorrect name? All are names as if in aqueous solution
4. Which of the following statements is not true of a weak acid?
5. What is the molarity of a solution of hydrochloric acid that was prepared by diluting 5.0 mL of 12 M HCl to 100 mL?
6. Which of the following statements are true of pH?
1. it is always less than 7 in the solution of an acid.
2. The pH of 0.1 M HCl is 1.
The pH of a 0.1 M solution of a weak acid is always less than 7 but more than 1.
7. The addition of solid sodium nitrite to a solution of nitrous acid will?
8. Which of the following solutions contains the smallest number of hydrogen ions?
9. Magnesium metal is added in exces to 50 mL of the solutions. From which would 0.10 g hydrogen be produced?
10. What is the concentration of hydroxide ion in 0.010 M HCl?