The test tube on the left contains 1 M HNO3, the middle one has 1 M HCl and the one on the right contains 1 M acetic acid.
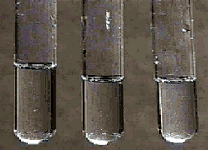
Magnesium metal has been added to each test tube.
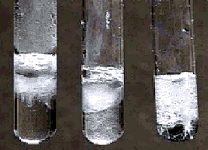
After a few minutes most of the magnesium in the HNO3 and HCl solutions has reacted. There is still magnesium metal on the bottom of the tube containing acetic acid.




