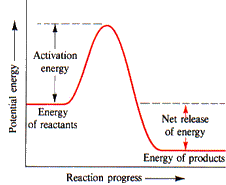
1. The reaction is endothermic.
2. The addition of a catalyst will raise the activation energy.
3. The reactants have less energy than the products.
4. All the molecules have enough energy to react.
Review Questions Chapter 13
1 Which of the following terms must have a negative sign for a reaction to
be spontaneous?
2. Which of the following statements are always true of a spontaneous reaction?
1. It always occurs rapidly.
2. It is always accompanied by an increase in temperature.
3. It is always exothermic.
3. Which of the following statements are correct in describing the reaction whose course is shown in the figure?

1. The reaction is endothermic.
2. The addition of a catalyst will raise the activation energy.
3. The reactants have less energy than the products.
4. All the molecules have enough energy to react.
4. Which of the following changes will increase the rate of the reaction shown in this equation?
4 NH3(g) + 5 O2(g)
4 NO(g) + 6 H2O(g)
1. increasing the concentration of oxygen
2. addition of a catalyst
3. increasing the temperature of the reaction
5. Which of the following statements is/are ture of a catalyst?
1. It increase the rate of a reaction
2. It can be recovered unchanged after the reaction ceases.
3. It prevents the reaction from reaching equilibrium.
6. For the equilibrium
2 P(s) + 3H2 2 PH3(g)
H
= -9.6 kJ
The equilibrium constant expression is:
7. Which of the following will not change the position of the equilibrium shown in Question 6?
8. Calculate the concentration of copper(II) ion is a saturated solution of copper(II) sulfide (Ksp of CuS = 8.5x10-45).
9. Which of the following silver salts is the most soluble?
10. An acid-base buffer may: