liquid
vapor
A system in equilibrium is a special situation where everything is in balance. Things rarely stay in balance; changes occur that shift the balance and the equilibrium that was present. We have discussed such changes in previous chapters. Recall the equilibrium that exists between a liquid and its vapor in a closed container:
liquid
vapor
At a given temperature, the vapor has a particular pressure; if the temperature is increased, it has a higher pressure. Increasing the temperature causes the equilibrium to shift to the right toward a higher concentration of vapor, but, if the system is maintained at that higher temperature, equilibrium will again be established.
In Chapter 12, we discussed the common-ion effect. We showed that, when acetate ion was added to a solution of acetic acid, the hydrogen ion concentration decreased. The addition of acetate ion caused the equilibrium
HC2H3O2
H+ + C2H3O2-
to shift to the left. The original equilibrium was upset by the addition of the acetate ion. When a new equilibrium was established, there were more acetate ions, fewer hydrogen ions, and more acetic acid molecules. But remember, both before and after the addition of acetate ions, the concentrations of acetic acid, hydrogen ion, and acetate ion were related by the equilibrium constant
| Ka = | [H+][C2H3O2-] [HC2H3O2] |
It is possible to predict how a particular stress or change in conditions will affect an equilibrium. Such predictions are based on a principle first stated by the French chemist Henri Le Chatelier (1850-1936). In 1888, Le Chatelier's Principle was proposed as follows: When a stress or change in conditions is applied to a system in equilibrium, the system shifts to absorb the effect of that stress.
In considering this principle, it is important to realize that equilibrium is not present while the change is taking place. The sequence is: The system is in equilibrium, the stress is applied, the system changes to absorb the stress, and finally equilibrium is again reached. The following sections discuss the effect of various stresses on equilibria.
A. The Effect of Concentration Changes on Equilibria
If the stress applied to a system in equilibrium is a change in the concentration of a component of the equilibrium, the system shifts to counteract that change. If the concentration of a substance is increased, the reaction that consumes that substance is favored, and the equilibrium shifts away from that substance. If the concentration of a substance is decreased, the reaction that produces that substance is favored, and the equilibrium shifts toward that substance.
To elaborate, an equilibrium is a combination of two reactions, one forward and one reverse. Changing the concentration of a reactant in an equation changes the rate of that reaction. When the concentration of a component in an equilibrium is increased, the rate of the reaction in which that substance is a reactant is increased; more of the product of that reaction is formed. When the concentration of a component is decreased, the rate of the reaction that uses that substance is decreased; less of its product is formed.
In studying the common-ion effect in, we calculated the effect of a concentration change on the equilibrium between acetic acid and its ions:
HC2H3O2
H+ + C2H3O2-
In 1.0 M acetic acid, the concentrations of both hydrogen and acetate ions
were calculated to be 4.2 X 10-3 M. We than raised the concentration
of acetate ions to 1.0 M by the addition of solid sodium acetate. How did this
change affect the equilibrium? The reaction that consumes acetate ion is the
one toward the left - the formation of acetic acid molecules. Every acetic acid
molecule formed uses up a hydrogen ion, which decreases the concentration of
hydrogen ions in the newly established equilibrium and increases the concentration
of acetic acid molecules. We calculated the concentrations in the new equilibrium
and found that the hydrogen ion concentration had decreased from 4.2 X 10-3
M to 1.8 X 10-5 M. The equilibrium had shifted to absorb the effect
of the stress caused by the increase in the concentration of acetate ions.
|
Example: Consider the equilibrium PCl3(g) + Cl2(g) Write the equilibrium constant expression for this reaction. Predict how the equilibrium position will be affected by a. an increase in concentration of PCl3 b. a decrease in concentration of Cl2 Solution The equilibrium constant expression for the reaction is
a. The reaction that consumes phosphorus trichloride is the forward reaction. Increasing the concentration of PCl3 will increase the rate of this reaction, decrease the concentration of chlorine, and increase the concentration of phosphorus pentachloride. The equilibrium will shift right. b. The reaction that produces chlorine is the one toward the left (the reverse reaction). If the concentration of chlorine is reduced, the rate of the reverse reaction will increase. The equilibrium will shift to produce chlorine and phosphorus trichloride. When equilibrium is reestablished, there will be less phosphorus pentachloride and more phosphorus trichloride present. Thus, decreasing the concentration of chlorine will decrease the rate of the forward reaction, and the equilibrium will shift left. |
B. The Effect of Pressure Changes on Equilibria
Involving Gases
A pressure change is a stress to those equilibria that involve gases - that is, those equilibria that have different numbers of gaseous molecules on the left and right sides of the equilibrium equation. Increased pressure favors the reaction that decreases the number of gaseous molecules.
In the equilibrium
N2(g) + 3 H2(g)
2 NH3(g)
the forward reaction produces two molecules of gas, whereas the reverse reaction produces four molecules of gas. In terms of moles, there are four moles of gas on the left of this equilibrium and two on the right. Recall that the volume of a gas is independent of the composition of the gas; that is, at the same temperature and pressure, one mole of any gas behaving ideally occupies the same volume as one mole of any other gas. Thus, the gases on the left occupy a total of four volumes, and those on the right occupy two volumes. Increased pressure decreases the volume available to this gaseous equilibrium and favors the forward reaction, because the forward reaction decreases the number of gaseous molecules. An increase in pressure on this equilibrium will favor the formation of more ammonia (Figure 13.9).
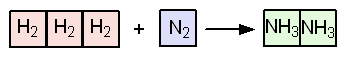
| FIGURE 13.9 Gaseous volumes in the N2, H2 and NH3 equilibrium. |
|
Example: Consider the following euilibria and predict how they will shift in response to increasing pressure.
Solution a. This equilibrium has two gaseous molecules on the left and one on the right. An increase in pressure will shift the equilbirium to the right, forming more PCl5. b. This equilibirium has two molecules of gas on the left and the same number on the right. An increase in pressure will not shift the equilibrium in either direction. |
C. The Effect of Temperature Changes on Equilibria
A change in temperature is a stress on a system in equilibrium. It changes the
rate of both forward and reverse reactions and at the same time changes the
value of the equilibrium constant. In every equilibrium, two reactions proceed
simultaneously, one forward and one reverse. One of these is endothermic (
H > 0), and one is exothermic (
H < 0). When the equilibrium is shown as an equation, the enthalpy term
H refers to the forward reaction. For example, in the hydrogen iodide equilibrium,
the forward reaction is exothermic, as shown by the equation
H2(g) + I2(g)
2HI(g)
H = -51.0 kJ
When the temperature of an equilibrium mixture is increased, the rate of both reactions increases , but the rate of the endothermic reaction (the reaction that absorbs the added energy) is increased more. For the hydrogen iodide equilibrium, an increase in temperature favors the endothermic reverse reaction. When the system returns to equilibrium, the hydrogen iodide concentration will be smaller and the concentrations of hydrogen iodine larger. The equilibrium constant will be changed:
| at 445°C, Keq = | [H]2
[H2][I2] |
= 64 | at 490°C, Keq = | [H]2
[H2][I2] |
= 45.9 |
|
Example: How will an increase in temperature affect the following equilibrium?
Solution In this equilibrium, the forward reaction (to form phosphorus pentachloride) is exothermic; the reverse reaction (to consume phosphorus pentachloride) is endothermic. An increase in temperature favors the endothermic reaction, and the equilibrium will shift to the left to absorb the added energy and produce more phosphorus trichloride. |
D. The Effect of Catalysts on Equilibria
A catalyst changes the rate of a reaction by providing an alternative pathway
with a lower activation energy. The lower-energy pathway is available to both
the forward and the reverse reactions of the equilibrium. The addition of a
catalyst to a system in equilibrium does not favor one reaction over the other.
Instead, it increases equally the rates of both the forward and the reverse
reactions. The rate at which equilibrium is reached is increased, but the relative
concentrations of reactants and products at equilibrium, and hence the equilibrium
constant, are unchanged.
|
Example: Given the equilibrium
a. Write the equilibrium constant expression for this reaction. b. How will the equilibrium shift if the temperature is increased? c. How ill the equilibrium shift if more bromine, Br2, is added to the reaction mixture? What will happen to the concentration of phosphorus tribromide, PBr3? d. How will an increase in pressure affect the relative concentrations of products and reactants? e. How will the addition of a catalysts affect this equilibrium f. Is the value of Keq increased, decreased, or unchanged by the change in conditions in parts b, c, and d? Solution a. The equilibrium constant expression is
b. The forward reaction is exothermic; the reverse reaction is endothermic. Increasing the temperature will increase the rate of both reactions but will increase more the rate of the reverse reaction, which is endothermic, thus changing the equilibrium constant. The concentration of phosphorus pentabromide will decrease; that of bromine and phosphorus tribromide will increase. c. If more bromine is added to the reaction mixture, the rate of the forward reaction, the one that consumes the added bromine, will be increased. There will be more phosphorus pentabromide and less phosphorus tribromide. d. In the forward reaction, two gaseous molecules combine to form one gaseous molecule. Increased pressure will favor the forward reaction. The concentrations of phosphorus tribromide and bromine will decrease; that of phosphorus pentabromide will increase. e. The addition of a catalyst will change neither the concentrations nor the value of Keq. The rate of both reactions will increase by the same amount. f. Keq will not be changed by the changes in conditions in parts c and d. Keq will be decreased by the temperature increase in part b. the reverse reaction is endothermic and favored by the increase in temperature. The reverse reaction uses up phosphorus tribromide; thus, the numerator of the equilibrium constant is decreased, the denominator increased, and the value of Keq decreased. |