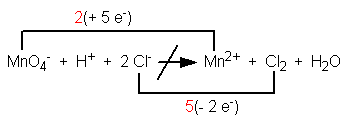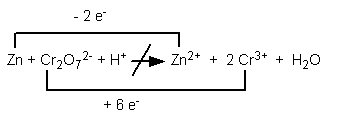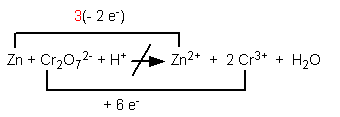Step 2. If the reaction occurs in acid, add hydrogen ion and water as reactants or products. If the reaction occurs in base, add hydroxide ion and water as reactants or products. Example: this reaction takes place in acid. We will leave H+ as a reactant and add water as a product, knowing we might need H+ as a product or water as a reactant.
Step 3. Assign an oxidation number to each element in the skeletal equation. Example:
Step 4. For those elements that change oxidation number, use a line above or below the equation to connect the reactant form with the product form. Write above or below the line the loss or gain of electrons that accompanies the change. Example:
Step 5. Using multipliers, equate the loss and gain of electrons. Example:
Step 6. Multiply the coefficient of the substance oxidized or reduced by the multiplier used to equate the electron changes. Example:
Balance the oxygen by changing the number of water molecules present. Balance the hydrogen ions to match the water molecules. Example: Each nitrate ion yields one NO2 and one O2-. Two nitrate ions yield two O2-. These require four hydrogens to form two water molecules, so the coefficient of H+ is changed to 4. The equation should now be balanced.
Step 7. Check that the charge is balanced. Example: the charge on the left is +2; the charge on the right is also +2. The equation is balanced.