Zn(s) + CuSO4(aq)
Cu(s) + ZnSO4(aq)
We have assumed that the oxidation-reduction reactions discussed so far have
taken place in a single container and that electrons are transferred directly
from the substance oxidized to the substance reduced. Although a redox reaction
may take place in this manner, it is also possible to transfer the electrons
through a wire or electrical circuit. Such an arrangement is called an electrochemical
cell.
An electrochemical cell that utilizes a spontaneous redox reaction to produce a flow of electrons is called either a galvanic cell, after the Italian chemist Luigi Galvani (1737-1798), or a voltaic cell, after Galvani's friend Alessandro Volta (1745-1827). Galvani and Volta were pioneers in the study of the production of electricity by chemical means. In a voltaic cell, the half-reactions of the spontaneous redox reaction are kept separate and the electrons produced flow through an external wire. Figure 14.1 shows one such cell.
The overall reaction of the cell shown is:
Zn(s) + CuSO4(aq)
Cu(s) + ZnSO4(aq)
In this apparatus, the two half-reactions are separated from each other by a porous membrane. On one side of the membrane is a solution of zinc sulfate and a piece of copper. A wire connects the zinc electrode and the copper electrode. At the zinc electrode the reaction is:
Zn
Zn2+ + 2 e-
The electrons produced in this half-reaction move through the wire to the copper-containing solution, where they combine with the copper ions to form copper atoms:
Cu2+ + 2 e-
Cu
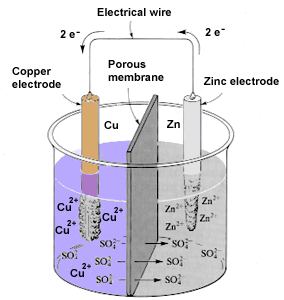
| FIGURE 14.1 An electrochemical cell that produces electricity. |
The excess sulfate ions left behind as the copper ions are reduced pass through the porous membrane to balance the charge of the newly formed zinc ions.
The electrons flowing through the wire are an electric current, and the arrangement or cell we have just described is a battery. The voltage of the cell can be calculated from the reduction potentials of the two half-reactions. In the cell of figure 14.1, the half reactions are:
Cu2+ + 2 e-
Cu E0 = 0.34 V
Zn
Zn2+ + 2 e- E0 = 0.76 V
The voltage of the cell (if the concentrations are 1 M) is 1.10 V.
Theoretically, any oxidation-reduction reaction can be used in a cell that
produces an electrical current. The batteries that we use in flashlights, calculators,
radios, and so on, produce electricity by various oxidation-reduction reactions.