Review Questions Chapter 9
1 Which sample occupies the smallest volume?
2. Which sample contains the most atoms?
3. In which sample do the particles have the highest average kinetic energy?
4. Which sample has the greatest mass?
5. Which sample has a density of 0.759 g/L?
6. In which sample do the molecules (or atoms in a monatomic sample) have the least attraction for one another?
7. Which sample has the highest melting point?
8. What volume of hydrogen measured at 0.95 atm and 27°C can be prepared by the reaction of 0.500 mol zinc with an excess of hydrochloric acid?
9. A 2.63 L sample of neon is collected at 730 torr and 26°C. What volume will the gas occupy at 1.10 atm and 10°C?
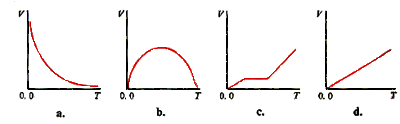
10. The volume of a gas sample at constant pressure is related to temperature T (in Kelvin) by the graph:
