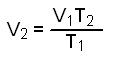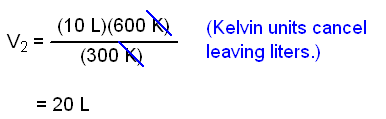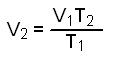
Sample Problem:
10 liters of oxygen in a piston at 300 K are warmed to 600 K. Its pressure
does not change. What is the new volume?
To calculate the final volume we will substitute the numerical values into this equation.
V1 = 10 L, T1
= 300 K, T2 = 600 K