| |
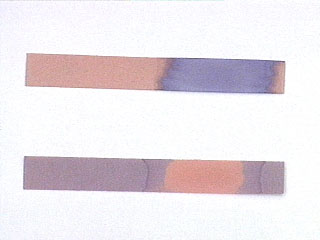
IMAGE. Red and blue litmus paper. Top: Red litmus paper with a drop of base placed on it (where the red paper has turned blue). Bottom: Blue litmus paper with a drop of acid placed on it (where the blue paper has turned red).
|

IMAGE. Universal paper is capable of indicating a range of pH values.
|
Litmus paper is a pH indicator paper coated with an organic dye which changes color in the presence of acids and bases. Litmus paper is used when determining whether a solution is acidic or basic. Litmus paper does not provide accurate information regarding the strength of the acid or base. There are two types of litmus paper: red and blue. Red litmus paper is used to check for basicity (red paper turns blue when exposed to bases), and blue litmus paper is used to check for acidity (blue paper turns red when exposed to acids). (Memory tool: blue for basic.)
Often, both types of litmus paper are used during the same experiment. A basic solution will wet a piece of blue litmus paper, and no color change will be observed. (The paper may appear bluer because it is wetted, but there will be no color change.) However, the lack of a color change does not conclusively indicate that the solution is basic since neutral solutions will not cause a color change. (Likewise, an acid will wet red litmus paper, with no color change observed.) Thus, when no color change is observed, the solution should be tested with the opposite color litmus paper to confirm acidity or basicity.
Alkacid paper, a universal indicator paper, can check for both acids and bases and indicate the approximate pH of a solution. The indicator will change color when wetted with an analyte solution, and the pH is approximated by matching the new color (where the sample has wetted the paper) to a standard color chart. Usually a color chart is available with the indicator paper dispenser, but a color chart can be prepared by wetting several pieces of indicator paper with a series of buffer solutions (solutions of known pH).
|