
What is Molecular Shape?
| Home |
| Table of Contents |
| Gateway Page |
| In this module: |
| Introduction |
| Familiar Chemicals
|
| Chemical Representations
|
| Exploring Molecular
Shape |
| Shape Name |
| Give it a Try! |
| Chemical Representations |
There are many ways to represent molecules, like those you just saw. Some of these ways are as follows:
MOLECULAR FORMULAS:
H2O, CO2, NH3, CH4
Molecular formulas represent a collection of atoms that are bonded together. These formulas DO NOT necessarily depict which atoms are bonded together, or even what type of bonds are involved in the molecule.
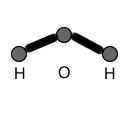
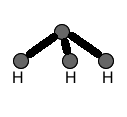
MOLECULAR STRUCTURES:
 |
 |
 |
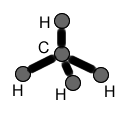 |
Molecular structures, unlike molecular formulas, DO show connectivitiy of atoms. In other words, these structures show which atoms are connected to which. They also reflect how many bonds exist between atoms. Also, molecular structures often show lone electron pairs on atoms. In contrast, they DO NOT show the geometry of a molecule.
3-D PICTURES (spatial representations):
Unlike molecular formulas, or molecular structures, 3-D pictures DO show geometries of molecules. However, they often do not often include lone pairs or depict multiple bonds between atoms, although they do show connectivity of atoms.
It is a goal of the Molecular Geometry portion of the tutorial to connect 3-D shapes to molecular formulas, as well as to familiarize you with the variety of 3-D shapes that molecules form.
|
Molecular Geometry |