GOALS:
When you have completed this module, you should be able to:
- Define entropy
- State the Second Law of Thermodynamics
- Describe how probability is the cause of the Second Law of Thermodynamics
- Use the Second Law of Thermodynamics to predict whether a reaction will be product- or reactant-favored.
A major goal of chemistry is predicting what reactions will occur and under what conditions. This question can be partially answered by thermodynamics. A reaction is called product-favored  if, after the reaction is over, there are more products than reactants. For example, the combustion of gasoline (mostly octane) in your car engine is a product-favored reaction: if, after the reaction is over, there are more products than reactants. For example, the combustion of gasoline (mostly octane) in your car engine is a product-favored reaction:
2 C8H18 (g) + 25 O2 (g) 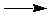 16 CO2 (g) + 18 H2O (g) 16 CO2 (g) + 18 H2O (g)
On the other hand, a reactant-favored reaction  is one in which reactants are more predominant than products. For example, the formation of sodium metal and chlorine gas from table salt is a reactant-favored process: is one in which reactants are more predominant than products. For example, the formation of sodium metal and chlorine gas from table salt is a reactant-favored process:
2 NaCl (s) 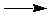 2 Na (s) + Cl2 (g) 2 Na (s) + Cl2 (g)
Two important things to remember about reactant-favored reactions: they can be forced to produce products if energy, such as heat, electricity, or some other form, is continuously supplied. Second, the reverse of every reactant-favored reaction is a product-favored reaction. For example the production of table salt from sodium metal and chlorine gas is a product-favored reaction:
2 Na (s) + Cl2 (g) 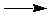 2 NaCl (s) 2 NaCl (s)
Early on, chemists noticed that most product-favored reactions were exothermic. The combustion of gasoline and the production of table salt, for example, have DHfº values of -10.452.3 kJ and -822.306 kJ, respectively.
There were some striking exceptions, however. For example, the melting of solid ice to liquid water at 25 ºC:
H2O (s) 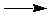 H2O (l) H2O (l)
is obviously product-favored, even though the reaction is endothermic. Another example is the mixing of ammonium chloride and barium hydroxide, which absorbs enough energy from the surroundings to freeze a beaker to a wet board (click on the image at right to see the reaction).

Ba(OH)2 • 8 H2O (s) + 2 NH4Cl (s) 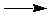 BaCl2 • 2 H2O (s) + 2 NH3 (aq) + 8 H2O (l) BaCl2 • 2 H2O (s) + 2 NH3 (aq) + 8 H2O (l)
Obviously, there is another factor than energy to consider when deciding if a reaction is product- or reactant-favored. How the energy and atoms are distributed in the reactants and products are just as important as the energy released or absorbed. The term used to describe this distribution is called entropy.
|