|
A 1.00 g antacid tablet is treated with an excess of hydrochloric acid and 0.32 g carbon dioxide is produced. What percentage of the tablet is calcium carbonate?
|
 |
What does "in excess" mean?
|
The first three steps of this problem are similiar to any stoichiometry problem. Work through the following steps. There is a table at the end of the page for you to enter your answers in.
 |
|
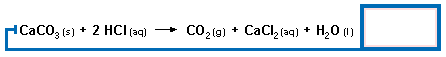 |
|
Click on formulas to see molecular weights.
|
Step 1:Convert grams of product to moles of product.
|
|
 |
How do I convert grams to moles?
|
Step 2:Calculate how many moles of the desired reactant would produce that amount of product using the balanced chemical equation.
|
|
 |
How do I convert a product to a reactant?
|
Step 3:Convert moles of reactant to grams of reactant.
|
|
 |
How do I convert moles to grams?
|
You can review the calculations for each CORRECT step above.
|