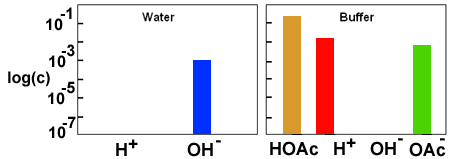
A few drops of base changes the pH of water by 3 or 4 pH units.

However, the same amount of base added to the buffer solution just converts some acetic acid to sodium acetate. Since the buffer concentration is higher than the added base the pH doesn't change very much.



