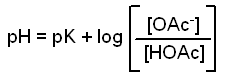
This equation relates the hydrogen ion concentration to the
concentration of acetic acid and sodium acetate in our buffer.
|
[H+] = K |
[HOAc]
[OAc-]
|
If we take the -log of both sides we get this new equation.
This gives us an equation that shows how the pH of the buffer
depends on the pK of the acid and the ratio of concentrations
of the anion and the acid.



