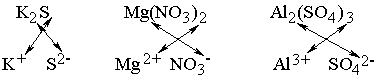Categories of Compounds
A compound is a chemical combination of elements. It has a constant composition
and a unique set of properties. Its composition is represented by its formula,
which lists the symbols of the elements it contains, with each symbol followed
by a subscript that tells how many atoms of that element are contained in the
simplest unit of the compound.
Some compounds exist as
molecules.
These molecular compounds usually contain only nonmetals. Table 6.1 lists several
molecular compounds,
their common names, and their melting points.
TABLE 6.1 Some molecular compounds
| Common name |
Formula |
Melting point, °C |
| acetone |
C3H6O |
-95 |
| ammonia |
NH3 |
-77 |
| cane sugar |
C12H22O11 |
185 |
| chloroform |
CHCl3 |
-63 |
| ethyl alcohol |
C2H5OH |
-117 |
| hydrazine |
N2H4 |
1.4 |
| phosgene |
Cl2CO |
-118 |
| water |
H2O |
0 |
Other compounds are ionic. These compounds are formed by the combination of ions.
(The names and formulas of ions were discussed in Section 5.7C). Ionic compounds
include hydroxides ( Section 5.7D ) and salts. A salt
is an ionic compound that contains a cation other than hydrogen and an anion other
than hydroxide. Many acids exist as ions in solution but as molecules in the pure
state.
Gratuitous Dust Explosion:

Click image for movie.
The formula of an ionic compound is neutral.
The ratio of the cations and anions it contains is such that
there is no excess charge. Thus, the combination of a sodium
ion, Na+, with a sulfate ion, SO42-,
to form sodium sulfate must be in a 2:1 ratio so that the resulting
compound is neutral, Na2SO4. The combination
of an aluminum ion, Al3+, with a chloride ion, Cl-,
to form aluminum chloride must be in a 1:3 ratio, giving the formula
AlCl3, which is neutral. The combination of an ammonium
ion, NH4+, with a phosphate ion, PO43-,
to form ammonium phosphate must be in a 3:1 ratio, giving the
neutral formula (NH4)3PO4. Notice
in this last formula that the ammonium ion is enclosed in parentheses
and followed by the subscript 3. This notation means that the
whole ion is taken three times. When a polyatomic ion is taken
more than once in a formula, it is enclosed in parentheses and
the number of ions contained in the formula is indicated by a
subscript following the parentheses. Monatomic ions and polyatomic
ions taken only once (for example, the sulfate ion in sodium sulfate)
are not enclosed in parentheses.
Notice two things about the examples shown below: In both magnesium nitrate
and aluminum sulfate, the polyatomic ion was enclosed in parentheses because
it was taken more than once; the monatomic ion was not enclosed in parentheses
regardless of its subscript. (2) If the charge on the two ions differs in magnitude,
the number of times the cation is taken equals the magnitude of the charge on
the anion. Similarly, the number of times the anion is taken equals the magnitude
of the charge on the cation. For example,