Naming
Compounds
Each compound has a name. Ideally, this name should indicate the composition
of the compound and perhaps something of its properties. Such names are called
systematic names and are based on a set of rules drawn up by IUPAC. Although
all compounds have systematic names, many also have trivial, or common, names.
Table 6.1 lists the common (trivial)
names of some molecular compounds. Several ionic compounds are listed in Table
6.2, with both their common and systematic names.
TABLE 6.2 Names and formulas of some common ionic
compounds.
| Common name |
Systematic name |
Formula |
| bleach |
sodium hypochlorite |
NaOCl |
| chalk |
calcium carbonate |
CaCO3 |
| lime |
calcium oxide |
CaO |
| milk of magnesia |
magnesium hydroxide |
Mg(OH)2 |
A. Oxidation Numbers
Many of the rules by which names are assigned are based on the concept of oxidation numbers. The oxidation number
of an element represents the positive or negative character (nature) of an atom of that element in a particular bonding situation. Oxidation numbers are assigned according to the following rules:
- The oxidation number of an uncombined element is 0. In the equation
Zn + 2 HCl  H2
+ ZnCl2
H2
+ ZnCl2
the oxidation number of zinc (Zn) as an uncombined atom is 0, and the
oxidation number of hydrogen in H2 is 0.
- The oxidation number of a monatomic ion is the charge on that ion. In ZnCl2,
the oxidation number of chlorine as Cl- is -1 and that of zinc
as Zn2+ is +2. In Ag2S, the oxidation number of silver
as Ag+ is +1 and that of sulfur as S2- is -2.
- Hydrogen in a compound usually has the oxidation number +1. An exception
to this rule occurs when hydrogen is bonded to a metal.
- Oxygen in a compound usually has the oxidation number -2. Peroxides are
an exception to this rule: In hydrogen peroxide, H2O2,
for example, the oxidation number of oxygen is -1.
- The sum of the oxidation numbers of the atoms in a compound is 0. For example,
in the compound ZnCl2, the oxidation number of the zinc ion is
+2 and that of each chloride ion is -1. The sum of these oxidation numbers
(+2 for zinc and -2 for the two chloride ions) is 0.
- In a polyatomic ion, the net charge on the ion is the sum of the oxidation
numbers of the atoms in the ion. We can use this rule to calculate the oxidation
number of nitrogen in the nitrate ion, NO3-, by setting
up the following equation:
| Oxidation number of nitrogen |
| +3 (oxidation number
of oxygen) = -1 |
| Oxidation number of Oxygen = -2 |
| By substituting, we get: |
| Oxidation number of
nitrogen +3(-2) = -1 |
| By rearranging, this equation becomes: |
| Oxidation number of
nitrogen = -1 - 3(-2) |
| = -1 + 6 = + 5 |


B. Binary Compounds
Many chemical compounds are binary; that is, they contain two elements. Binary
compounds are of several varieties.
1. Binary compounds containing
a metal and a nonmetal
Binary compounds of a metal and a nonmetal contain a metallic cation and a nonmetallic
anion. The names and formulas of cations and anions were introduced in Section
5.7 (Tables 5.7-5.9). Recall that the
alkali metals form only ions with a +1 charge, the alkaline earth metals form
only ions with a +2 charge, and aluminum forms only the ion Al3+.
For these ions, the name of the element followed by the term ion is an unambiguous
name. For example, the sodium ion can only be Na+, the calcium ion
only Ca2+. According to IUPAC rules, the names of all other metallic
cations contain the name of the element followed by its oxidation state (in
parentheses) in that ion. This rule prevents ambiguity. The name chromium
ion does not say whether the ion is Cr2+ or Cr3+;
the proper names for these ions are chromium(II) and chromium(III). The anions
in binary compounds are named by using the root name of the element, followed
by the suffix ide; for example, bromide ion is Br-,
the sulfide ion is S2-, and the oxide ion is O2-.
In these examples, the root name of the element is italicized. For binary compounds,
the cation is named first and the anion second. Thus,
NiCl2 is nickel(II) chloride
K2S is potassium sulfide
CaBr2 is calcium bromide
ZnO is zinc(II) oxide
Before leaving this group of compounds, we should mention again the second and less-preferred method of naming cations of the same element in different oxidation states. This older method gives the ending ous to the ion of lower oxidation state and the ending ic to the ion of higher oxidation state. Often this system also uses the Latin root of the name of the element. Thus, in this system, Fe2+ is ferrous and Fe3+ is ferric; Pb2+ is plumbous and Pb4+ is plumbic. These elements that use Latin roots are shown in Table 6.3.
TABLE 6.3 Some elements with non-English rootnames (root is italicized)
| Element |
Latin name |
|
Element |
Latin name |
| copper |
cuprum |
lead |
plumbum |
| gold |
aurum |
silver |
argentum |
| iron |
ferrum |
tin |
stannum |

2. Binary compounds containing two nonmetals
but not hydrogen
Binary compounds of two nonmetals, neither of which is hydrogen, are molecular
rather than ionic. They do not contain cations and anions. Carbon dioxide (CO2)
and phosphorus trichloride (PCl3) are examples of such compounds.
They are named using prefixes to state how many atoms of an element are in one
molecule of the compound. (The prefixes are listed in Table 6.4.)
TABLE 6.4 Prefixes used in naming binary compounds of two nonmetals
Number
of atoms |
Prefix |
|
Number
of atoms |
Prefix |
|
Number
of atoms |
Prefix |
| 1 |
mono- |
5 |
penta- |
9 |
nona- |
| 2 |
di- |
6 |
hexa- |
10 |
deca- |
| 3 |
tri- |
7 |
hepta- |
11 |
hendeca- |
| 4 |
tetra- |
8 |
octa- |
12 |
dodeca- |
The name of the second element is modified to the root of its name followed by the ending ide. In both the formula and the name of these compounds, the most nonmetallic element comes first (see
Figure 5.16
in Chapter 5). The prefix mono is often omitted for the first element but never omitted for the second. Thus,
CO is carbon monoxide
SF6 is sulfur hexafluoride
N2O is dinitrogen monoxide

3. Binary acids
The binary compound formed when a halogen or any element, except oxygen, from
Group 6 of the periodic table combines with hydrogen can be named as were
the binary nonmetallic compounds discussed in the preceding section. However,
when these compounds are dissolved in water, the solution contains hydrogen
ions. Because this property identifies an acid ( Section 5.7D ), these compounds
must also be named as acids. Therefore, these compounds have two sets of names,
one for the pure state and one for the compound dissolved in water (see Table
6.5). Two points should be noted: (1) The acid name has the prefix hydro
and the suffix ic. (2) These formulas are always written with hydrogen
first. Other nonmetals form compounds with hydrogen, but they are not
acids; their formulas are written with hydrogen last. Methane, CH4,
ammonia, NH3, and arsine, AsH3, are examples.
TABLE 6.5 Nomenclature for binary acids
| Formula |
Name in pure state |
Name in water solution |
| HCl |
hydrogen chloride |
hydrochloric acid |
| H2S |
hydrogen sulfide |
hydrosulfuric acid |
| HBr |
hydrogen bromide |
hydrobromic acid |

4. Pseudo-binary compounds
Several polyatomic ions act so much like monatomic ions that they are classified
as such. These ions are called pseudo-binary ions. They include the ammonium
ion, NH4+, the hydroxide ion, OH-, the cyanide
ion, CN-, and others. Compounds containing these ions are pseudo-binary
compounds.
The properties of the ammonium ion are much like those of the alkali-metal ions.
Compounds containing the hydroxide ion are bases. A general definition of a base is that its aqueous solution contains more hydroxide than hydrogen ions. (Bases were introduced in
Section 5.7D.)
The cyanide ion behaves very much like a halogen ion. Many compounds containing the cyanide ion are extremely toxic.
C. Ternary Compounds
Ternary compounds are those compounds containing three elements. Ionic ternary
compounds are formed by the combination of a monatomic cation with a polyatomic
(containing several atoms) anion, as in sodium nitrate, NaNO3. A
polyatomic anion is derived from a ternary acid.

1. Ternary acids and
their anions
When a ternary compound contains hydrogen and a polyatomic anion (for example, HNO3), its name in the pure state is hydrogen followed by the name of the anion. Pure HNO3 has the name hydrogen nitrate. When this compound is dissolved in water, it is an acid and is named as such. HNO3 in water solution is named nitric acid. Table 6.6 lists the formulas of some of these compounds, the names they carry when in water solution, the oxidation number of the nonmetal other than oxygen that they contain, and the name and formula of their anions. The rules for naming these compounds as acids follow the table. Be sure to study the table as you read the rules and notice the pattern shown in the names and formulas.
The rules for naming ternary acids are as follows:
- The name of the most common oxyacid for a particular
nonmetal is the root of the element's name plus the suffix ic. It has
no prefix. The name of the anion of this acid is the root of the element's
name plus the suffix ate. The oxidation number of the nonmetal in this
acid is high but not necessarily the highest possible. These acids are sometimes
referred to as ic-ate acids. Of the oxyacids in Table 7.6, nitric,
sulfuric, phosphoric, and chloric are in the category of "most common." In
the table, the formulas marked with an asterisk are the most common acids
for a particular nonmetal.
- The name of the acid in which the nonmetal has the
next lower oxidation number is the element's root plus the suffix ous.
The name of its anion is the root plus ite. These acids may be called
ous-ite acids. Of the acids in Table 6.6, nitrous, sulfurous, and chlorous
fall into this group. Their formulas can be predicted if you have learned
the formulas in the first group. The anion of an ous-ite acid contains
one fewer oxygen atom than that of the ic-ate acid.
- As with the halogens, if there is an oxyacid in which
the nonmetal has an even lower oxidation number, that acid is named using
the prefix-suffix hypo-ous, and its anion using hypo-ite. Of
the acids in Table 6.6, only hypochlorous is in this category. Its formula
can be predicted if you know the formula of chloric acid. The anions of these
acids contain two fewer oxygen atoms than the anions of the ic-ate
acids.
- Again as with the halogens, if there is an oxyacid
in which the nonmetal has a higher oxidation number than in the most common
acid, that acid is named per-ic acid and its anion per-ate.
Of the acids in Table 6.6, only perchloric is in this group. Its formula can
be predicted from the formula of chloric acid. The anion will contain one
more oxygen atom than the anion of the ic-ate acid.
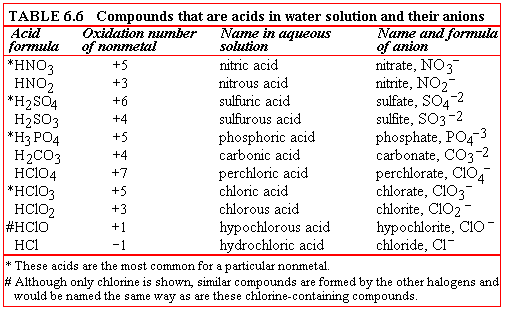
The formulas of the salts of these acids are neutral combinations of ions (discussed in
Section 6.1).
To name them, first name the cation according to the rules given in
Section 6.2B1.
The names of the anions are given in Table 6.6.
2. Ternary acids containing carbon
Many acids contain only carbon, hydrogen, and oxygen. Acetic acid is an example.
Its formula can be written as
HC2H3O2 or HCH3CO2
or CH3COOH or CH3CO2H
Regardless of how it is written, there is only one acidic hydrogen
in acetic acid; the other three hydrogens do not separate as hydrogen ions in
aqueous solution. Notice how the acidic hydrogen is placed by itself in each
of the formulas to signify this difference. Many acids, like acetic acid, contain
a group of atoms bonded to a -COOH group. Only the hydrogen of the -COOH group
is an acidic hydrogen. For example,
C6H5COOH benzoic acid
C2H3COOH acrylic acid
These acids are called carboxylic acids. They are discussed more fully in
Chapter 15.
In naming the anions of these acids, the ic of the acid is replaced by ate. Thus,
| acetic acid HC2H3O2, yields acetate ion, C2H3O2- |
| benzioc acid C6H5COOH, yields benzoate ion, C2H5COO- |
| acrylic acid C2H3COOH, yields acrylate ion, C2H3COO- |
3. Salts containing more than one cation
Occasionally you will encounter a salt containing more than one cation. If both cations are metals, they are named in the order in which they are written according to the rules already given. If one of the cations is hydrogen, the salt can be named either by calling the cation hydrogen or by adding bi as a prefix to the name of the anion. Thus, NaHCO3 can be named sodium hydrogen carbonate or sodium bicarbonate. Salts with more than one cation, one of which is hydrogen, are sometimes called acid salts.
H2 + ZnCl2