Real Gases
Thus far in this chapter, we have assumed that all gases are ideal and behave in accordance with the postulates of the kinetic molecular theory and the ideal gas equation. Under standard conditions of temperature and pressure, and also at higher temperatures and lower pressures, the behavior of most real gases such as oxygen, nitrogen, and carbon dioxide is that predicted by the gas laws and the kinetic molecular theory. It is for this reason that we study the properties of ideal gases. However, as the temperature of a gas is decreased, the kinetic energy of the molecules decreases, their movement becomes more sluggish, and the attractive forces that exist between real molecules play a larger role in determining the behavior of the sample. Likewise, if the pressure is increased and the volume decreased until the volume of the space between the molecules approximates the volume of the molecules themselves, the molecules can no longer act as the wholly independent particles postulated by the kinetic molecular theory.
Under these conditions of low temperature and high pressure, any attractive forces that exist between the molecules of the gas come into play. These attractive forces are dipole-dipole interactions and dispersion forces.
Dispersion forces also called London or Van der Waal's forces, are weak forces of attraction that exist between all molecules without regard to the polarity of the molecules. To understand the nature of these forces, we need to remember that, even though a molecule may have no permanent dipole, it does have a cloud of rapidly moving electrons. If this cloud is distorted, no matter how briefly, the molecule will then have a temporary negative charge at one end and a temporary positive charge at the other end (Figure 9.12). In other words, the molecule has a temporary dipole. This temporary dipole can distort the electron clouds of nearby molecules so that they, too, have temporarily induced dipoles. The forces of attraction between the temporary partial positive charges on some molecules and the temporary partial negative charges on neighboring molecules are the dispersion forces.
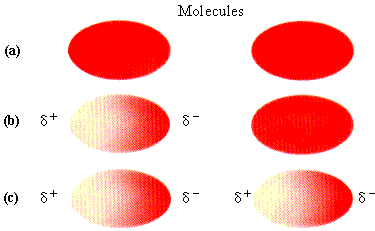
| FIGURE 9.12 The development of temporary dipoles in molecules: (a) electron clouds with charge evenly dispersed; (b) temporary distortion of left cloud, causing a temporary dipole; (c) induced distortion of right cloud caused by presence of dipole in left cloud, also resulting in a temporary dipole.
|
Under standard conditions of temperature and pressure, molecules move freely without intermolecular attraction, as illustrated in Figure 9.13a. In Figure 9.13b, the molecules are moving more slowly, they are closer together, and they interact. The dipole in one molecule, regardless of whether it is real or temporary, interacts with the dipole of its neighbors. The lower the temperature and the closer the molecules are together (a result of higher pressure), the more effective are these dipole-dipole interactions in preventing the free movement of molecules required by the kinetic molecular theory. Gases that show these tendencies are said to be real gases, as opposed to ideal gases (those whose behavior is close to that predicted by the gas laws). Gases of low molecular weight and no polarity are the most ideal--for example, hydrogen and helium.
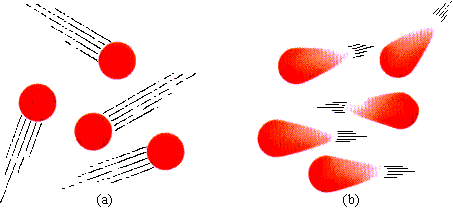
| FIGURE 9.13 The interaction of polar molecules: (a) gas molecules move freely at STP, without interaction; (b) interaction occurs when the gas is at low temperature or high pressure, causing temporary dipoles.
|
We expect the behavior of real gases to deviate more and more from the ideal as the polarity (either real or induced) and the molecular weight of the molecules increase. Molecular weight is a factor because the size and mass of a molecule increase as its molecular weight increases.