
IMAGE. A Spectronic 20™.
|
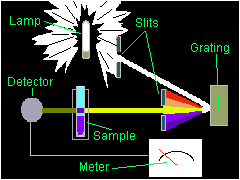
IMAGE. Schematic diagram of a Spectronic 20™
(Click image for larger view)
|
The Spectronic 20™ is used to measure the absorbance (or transmittance) of solutions. A Spectronic 20™ is capable of measuring % transmittance and absorbance over the range of 340 to 950 nm (the range 600 to 950 nm requires a special infrared filter and a different lamp). Data collected with spectrophotometer can be used to perform both qualitative and quantitative analyses. This set of web pages describes the use of a spectrophotometer to perform quantitative analyses.
A spectrophotometer can be used to quantitatively establish the Beer-Lambert relationship for analytes that absorb in the spectrophotometer's range. (Recall, the Beer-Lambert Relation: A = elc Once the Beer-Lambert relationship is established, data can be collected for the determination of:
- the molar extinction coefficient (e)
- the path length of the sample cell (l)
- the concentration of an analyte in solution (c)
- the predicted absorbance of a solution of known concentration (A)
The spectrophotometer is a relatively simple instrument. The spectrophotometer consists of a light source (lamp), a grating, slits, a sample compartment, and a detector.
|