Cofactors
In some of the proteins you have seen already, there have been other molecules bound, either covalently or noncovalently, to the proteins. These accessory molecules are called cofactors
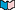 , and do specific things to help the proteins carry out their functions. For example, in hemoglobin (lower left), the cofactor heme binds the oxygen that is transported from the lungs to the other tissues of the body; the second image is zoomed in on one of the heme molecules. In alcohol dehydrogenase (third image), which is responsible for oxidizing alcohol to acetate in the liver, the cofactors nicotinamide adenine diphosphate (NAD), dimethyl sulfoxide (DMS) and Zn2+ work together to remove electrons from molecules of alcohol. The final image shows a close-up of the NAC, DMS and Zn2+ cofactors.
, and do specific things to help the proteins carry out their functions. For example, in hemoglobin (lower left), the cofactor heme binds the oxygen that is transported from the lungs to the other tissues of the body; the second image is zoomed in on one of the heme molecules. In alcohol dehydrogenase (third image), which is responsible for oxidizing alcohol to acetate in the liver, the cofactors nicotinamide adenine diphosphate (NAD), dimethyl sulfoxide (DMS) and Zn2+ work together to remove electrons from molecules of alcohol. The final image shows a close-up of the NAC, DMS and Zn2+ cofactors.
|
Quaternary Structure
The fourth level of protein structure, quaternary structure
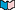 , arises when more than one protein chain is present in the active protein. For
example, the hemoglobin in your blood (shown at right) is composed of four chains which work together as two subunits (blue and yellow). When oxygen binds to one subunit of hemoglobin, the interactions between the subunits change. In other words, oxygen binding
affects the quaternary structure. Quaternary structure is the usual mechanism
for the processes known as allostery (in which binding of a molecule at one site
on a protein has effects at other sites far away from that site) and cooperativity
(in which binding of a molecule at one site in a multisubunit protein increases
or decreases the likelihood that another molecule will bind at another site of
the protein).
, arises when more than one protein chain is present in the active protein. For
example, the hemoglobin in your blood (shown at right) is composed of four chains which work together as two subunits (blue and yellow). When oxygen binds to one subunit of hemoglobin, the interactions between the subunits change. In other words, oxygen binding
affects the quaternary structure. Quaternary structure is the usual mechanism
for the processes known as allostery (in which binding of a molecule at one site
on a protein has effects at other sites far away from that site) and cooperativity
(in which binding of a molecule at one site in a multisubunit protein increases
or decreases the likelihood that another molecule will bind at another site of
the protein).
|
|