State Functions and Hess' Law
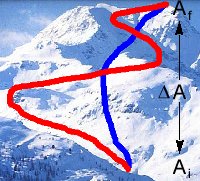 When you are climbing a mountain, do you take the short, steep route or the long, not as steep route? In the first case, you might make it to the top sooner, but the climb would be a lot harder. In the second case, you might be climbing longer, but the going will probably be easier. In both cases, however, the end result is the same: you're at the top of the mountain. The vertical distance you climbed is equal to the elevation at the top of the mountain minus the elevation at the bottom where you started. Mathematically, you could write this as DA = Af - Ai, where A represents altitude, Af represents your final altitude at the top of the mountain, and Ai represents your initial altitude at the bottom of the mountain. DA depends only on the initial and final states, not on the route you took to get there.
When you are climbing a mountain, do you take the short, steep route or the long, not as steep route? In the first case, you might make it to the top sooner, but the climb would be a lot harder. In the second case, you might be climbing longer, but the going will probably be easier. In both cases, however, the end result is the same: you're at the top of the mountain. The vertical distance you climbed is equal to the elevation at the top of the mountain minus the elevation at the bottom where you started. Mathematically, you could write this as DA = Af - Ai, where A represents altitude, Af represents your final altitude at the top of the mountain, and Ai represents your initial altitude at the bottom of the mountain. DA depends only on the initial and final states, not on the route you took to get there.
Scientists have a name for processes like this. In this case, A would be called a state function  . Many thermodynamic quantities, including temperature, energy, and enthalpy, are state functions because changes in their values depend only on their initial and final values. . Many thermodynamic quantities, including temperature, energy, and enthalpy, are state functions because changes in their values depend only on their initial and final values.
Because these values are state functions, you can combine two or more thermochemical equations to find changes in thermodynamic values that you don't know directly. For example, an ancient way of producing lead metal (Pb (s)) from its ore, galena (PbS (s)) is a two step process. First, the galena is cooked in air to form lead oxide:
PbS (s) + 3/2 O2 (g)  PbO (s) + SO2 (g) DH1 = -413.7 kJ PbO (s) + SO2 (g) DH1 = -413.7 kJ
Second, the lead oxide is treated with coal (C(s)) to form metallic lead and carbon monoxide:
PbO (s) + C (s)  Pb (s) + CO (g) DH2 = 106.8 kJ Pb (s) + CO (g) DH2 = 106.8 kJ
What is the enthalpy change for the overall process? It's just the sum of the enthalpy changes of each step:
PbS (s) + C (s) + 3/2 O2 (g) 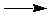 Pb (s) + CO (g) + SO2 (g) Pb (s) + CO (g) + SO2 (g)
DH = DH1 + DH2 = -306.9 kJ
This is an example of Hess' Law: if the equation for a reaction can be written as the sum of two or more other reactions, the DH of the overall reaction is the sum of the DH's of the other reactions.
If you know the DH's of some reactions, you can use the rules of thermochemical equations and Hess' Law to determine the DH of many other reactions.
|