Standard Enthalpies of Formation
As mentioned on the previous page, using Hess' Law makes it possible to calculate many DH's from just a few reactions for which DH is known. Scientists have compiled a long list of standard enthalpies of formation 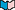 (DHfº) for this purpose. Each DHfº corresponds to a special thermochemical equation with the following features. (DHfº) for this purpose. Each DHfº corresponds to a special thermochemical equation with the following features.
1. One mole of a compound is formed from its elements.
For example, C(s) + O2 (g) 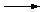 CO2 (g) would define the DHfº for carbon dioxide. 2Na (s) + Cl2 (g) CO2 (g) would define the DHfº for carbon dioxide. 2Na (s) + Cl2 (g) 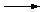 2NaCl (s) would not define the DHfº for sodium chloride, because two moles of NaCl (s) are being formed. MgO (s) + H2O (l) 2NaCl (s) would not define the DHfº for sodium chloride, because two moles of NaCl (s) are being formed. MgO (s) + H2O (l) 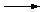 Mg(OH)2 (s) would not either, because Mg(OH)2 is being formed from MgO and H2O, which are not elements. Mg(OH)2 (s) would not either, because Mg(OH)2 is being formed from MgO and H2O, which are not elements.
2. The reactants must be in their standard states.
The standard state of an element or compound is the form it is in under standard conditions (25 ºC and 1 atm of pressure). H2 (g) is the standard state of hydrogen, rather than H2 (l), because hydrogen gas is found at 25 ºC and 1 atm instead of liquid hydrogen. Some substances can be found in more than one form under standard conditions: oxygen can be dioxygen (O2 (g)) or ozone (O3 (g)) and carbon can be graphite, diamond, or buckminsterfullerene. In these cases, the most common form (e.g. O2 (g) and graphite) is chosen as the standard state.
3. The DHfºs of elements in their
standard states are defined as zero.
This is because the reactants in thermochemical equations defined by DHfº are elements in their standard state. If the reactants and products are the same, there is no change, so the change in enthalpy must be zero.
 Click on the circles in the diagram below to see how DHfºs
can be used to find the DHº for the process
of photosynthesis:
Click on the circles in the diagram below to see how DHfºs
can be used to find the DHº for the process
of photosynthesis:
6 CO2(g) + 6 H2O(g)  C6H12O6(s) + 6 O2(g) C6H12O6(s) + 6 O2(g)
|