 |
|
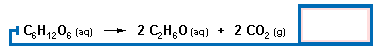 |
|
Click on formulas to see molecular weights.
|
 If 150.0 g glucose are initially present, what is the theoretical yield
(in grams) of ethanol?
If 150.0 g glucose are initially present, what is the theoretical yield
(in grams) of ethanol?
Follow these three steps. After the steps are listed, there is a table for you to enter your answers from each step.
Step 1: Convert grams of glucose to moles of glucose.
|
|
 |
How do I convert grams to moles?
|
Step 2: Calculate how many moles of ethanol can be produced from the number of moles glucose in Step 1.
|
|
 |
How do I convert a reactant to a product?
|
Step 3: Convert moles ethanol to grams of ethanol.
|
|
 |
How do I convert moles to grams?
|
|