|
Chemists calculate the yields of reactions because the percent yield gives an indication of the efficiency of the reaction. The closer the actual yield is to the theoretical yield, the closer the percent yield will be to 100 %. The closer the percent yield is to 100 %, the more effiecient the reaction. In an industrial setting, it is economically important to have efficient reactions so maximum products are formed.
The alcohol in wine is produced when the glucose in fruit ferments to ethanol:
 |
|
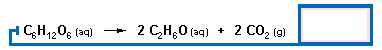 |
|
Click on formulas to see molecular weights.
|
 If 150.0 g glucose are initially present, what is the theoretical yield
(in grams) of ethanol?
If 150.0 g glucose are initially present, what is the theoretical yield
(in grams) of ethanol?
|