|
|
Step 1: Construct an ICE Table.
How many grams of NaNO3 are produced when 5.3 grams of Na2CO3 are added to 250.0 mL of 0.50 M HNO3 and the reaction is allowed to go to completion?
| Na2CO3 (aq) |
+ |
2 HNO3 (aq) |
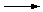 |
2 NaNO3 (aq) |
+ |
CO2 (g) |
+ |
H2O (l) |
The balanced chemical reaction is placed at the top of the table. Each reactant and each product is the heading for a column in the table.
Along the left side of the table, label the rows: Initial amount, Initial (moles), Change (moles), End (moles), and End amount:
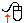 |
Move mouse over row headings in the above table to see brief definition.
|
|
Na2CO3 |
+ |
2 HNO3 |
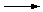 |
2 NaNO3 |
+ |
CO2 |
+ |
H2O |
| Initial amount |
Quantity of reactant or product present before the chemical reaction takes place measured as mass or volume. |
| Initial (moles) |
Number of moles of reactant or product present before the chemical reaction takes place. |
| Change (moles) |
This is the amount by which the number of moles reactant or product changes over the course of the chemical reaction. As a chemical reaction occurs, the amount of reactants decrease and the amount of products increase as reactants are converted to products. Therefore, the change in the reactants will be negative and the change in the products will be positive. |
| End (moles) |
Number of moles of reactant or product present after the chemical reaction takes place. |
| End amount |
Quantity of reactant or product present after chemical reaction takes place measured as a mass or volume. |
The middle three rows (Initial (moles), Change (moles), and End (moles)) will be completed for every problem. This is where the name ICE Table comes from. Some problems may not require the Initial amount and End amount. They can either be left off the table or left empty.
|