|
Step 2: Decide what information about the problem is known and insert in ICE Table.
How many grams of NaNO3 are produced when 5.3 grams of Na2CO3 are added to 250.0 mL of 0.50 M HNO3 and the reaction is allowed to go to completion?
| Na2CO3 (aq) |
+ |
2 HNO3 (aq) |
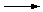 |
2 NaNO3 (aq) |
+ |
CO2 (g) |
+ |
H2O (l) |
The next step is to place any known information into the ICE Table.
In this problem, we are given the initial amounts of sodium carbonate (in grams) and nitric acid (in milliliters). We can also assume that there are no products present before the reaction takes place.
|
Na2CO3 |
+ |
2 HNO3 |
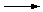 |
2 NaNO3 |
+ |
CO2 |
+ |
H2O |
| Initial amount |
5.3 g |
|
250.0 mL |
|
0 |
|
0 |
|
0 |
| Initial (moles) |
| Change (moles) |
| End (moles) |
| End amount |
|