Formula
Weights
A. Calculation of Formula Weights
The formula weight (sometimes called the molecular weight) of a compound or
ion is the sum of the atomic weights of all elements in the compound or ion,
with each element's atomic weight being multiplied by the number of atoms of
that element occurring in the formula.

B. Moles of Compounds
In Section 4.4 the mole was defined
as 6.02 X 1023 items. In that section, we talked about moles of atoms
and you learned that one mole of a particular element has a mass in grams equal
to the element's atomic weight. We can also have moles of compounds or of ions.
One mole of a compound or an ion will
have a mass in grams equal to the formula weight of that compound or ion. In
Example 6.8 we calculated the formula weight of sulfuric acid to be 98.09 amu,
thus it follows that one mole of sulfuric acid has a mass of 98.09 g. The formula
of a compound also tells how many moles of a particular element are contained
in one mole of the compound. For example, one mole of sulfuric acid contains:
2 mol hydrogen atoms, weighing 2(1.008 g) or 2.016 g
1 mol sulfur atoms, weighing 1(32.07 g) or 32.07 g
4 mol oxygen atoms, weighing 4(16.00 g) or 64.00 g
Note that these individual masses add up to our calculated formula weight
for the acid. This relationship between mass and moles of a compound is often
used as a conversion factor in solving problems.

|
Example:
How many atoms of oxygen are in 0.262 g carbon dioxide, CO2?
Wanted: ? atoms oxygen
Given: 0.262 g carbon dioxide
Conversion factors
The formula weight of carbon dioxide is:
carbon 1x12.01 = 12.01
Oxygen 2x16.00 = 32.00
Total: 44.01
amu (for CO2)
Equation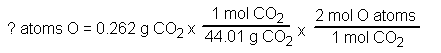

Answer: 7.17x1023 atoms O |
In this example , notice that each factor involving a mole states the chemical
composition of the mole: "1 mol CO2" and "2 mol O atoms." As problems
become increasingly complex, this bookkeeping habit becomes especially important.
Notice also that the example deals with atoms of oxygen; we are not concerned
here with the fact that oxygen exists in nature as a diatomic molecule, O2.

C. Percent Composition
Percent means parts per hundred. The
percent composition of a compound is the number of grams of each element or group of elements in 100 g of the compound, expressed as a percent.
| |
Percent element |
= |
g element
g compound |
X |
100% |
For example, the percent composition of potassium chloride, KCl, can be calculated from the atomic weights of potassium and chlorine and the formula weight of KCl.
Formula weight of KCl: 39.10 g + 35.45 g = 74.55 g
| |
Percent potassium: |
39.10 g K
74.55 g KCl |
X 100% = 52.45% potassium |
| |
Percent chlorine: |
35.45 g Cl
74.55 g KCl |
X 100% = 47.55% chlorine |
|
Example:
Calculate the percent composition of carbon tetrachloride, CCl4
Solution
The formula weight of carbon tetrachloride is:
|
carbon
|
12.01 x 1 =
|
12.01 |
|
chlorine
|
35.45 x 4 =
|
141.8 |
| |
|
153.8 amu |
| Percent C |
12.01 g C
153.8 g CCl4
|
x100% = 7.81%C |
| Percent Cl |
141.8 g Cl
153.8 g CCl4
|
x100% = 92.19%Cl |
It is always wise to chck these percent calculations by assuring yourself
that they add up to 100% as they do here.
|