Definition and Functional Groups
Carbohydrates
 are either organic compounds with the general chemical formula Cx(H2O)y,
or other molecules derived from them. Some examples of carbohydrates are glyceraldehyde
(C3H6O3, x = 3, y = 3), glucose (the main sugar
found in your blood and the energy source for your brain, C6H12O6,
x = 6, y = 6) and sucrose (table sugar, C12H22O11,
x = 12, y = 11).
are either organic compounds with the general chemical formula Cx(H2O)y,
or other molecules derived from them. Some examples of carbohydrates are glyceraldehyde
(C3H6O3, x = 3, y = 3), glucose (the main sugar
found in your blood and the energy source for your brain, C6H12O6,
x = 6, y = 6) and sucrose (table sugar, C12H22O11,
x = 12, y = 11).

How did carbohydrates get their name? Type your answer in the space given, then click on the check button to see the answer.
|
|
19th century chemists thought
carbohydrates were simple combinations of carbon (carbo-)
and water (-hydrates). They soon discovered, however, that
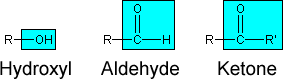
the primary functional groups in carbohydrates were not water but
hydroxyl, aldehyde, and ketone groups instead. (Remember that R
can refer to any organic group. In the ketone group, the prime after
the R on the right simply means that that organic group can be different
from the other one.)
 From
the following list of compounds, pick the one(s) that could be carbohydrates.
Click on the Check box when you are done. From
the following list of compounds, pick the one(s) that could be carbohydrates.
Click on the Check box when you are done.
|