Biomolecules:
DNA 1
| Home |
| Table of Contents |
| Biomolecules Gateway Page |
| Jmol Tutorial |
In this module: |
| Introduction |
| Nucleotides |
| Polymerization of Nucleotides |
| Base Pairing 1 |
| Base Pairing 2 |
| Complementary Sequences |
| Replication |
Polymerization of Nucleotides (Phosphodiester Bonds)Nucleotides are joined together similarly to other biological molecules, by a condensation reaction that releases a small, stable molecule. Unlike proteins, carbohydrates, and lipids, however, the molecule that is released is not water but pyrophosphate (two phosphate groups bound together). When pyrophosphate is cleaved by the addition of water, a great deal of free energy is released, ensuring that the reverse process (hydrolysis of the phosphodiester bond to give free nucleotides) is very unlikely to occur.
|
||
|
1. |
The 5' group of a nucleotide triphosphate is held close to the free |
|
|
2. |
The 3' hydroxyl group forms a bond to the phosphorus atom of the free nucleotide closest to the 5' oxygen atom. Meanwhile, the bond between the first phosphorus atom and the oxygen atom linking it to the next phosphate group breaks. |
|
|
3. |
A new phosphodiester bond now joins the two nucleotides. A pyrophosphate group has been liberated. |
|
|
4. |
The pyrophosphate group is hydrolyzed (split by the addition of water), releasing a great deal of energy and driving the reaction forward to completion. |
|
|
Why do the nucleotides in DNA have a hydrogen atom at the 2' carbon instead of the hydroxyl group in ribose? The answer is that a hydroxyl group at the 2' position can participate in a reaction that cleaves the phosphodiester bond. (To see how, go to the Ribozymes page in the Enzymes module. Just close that window when you are done and ready to return to this module.) Since this hydroxyl group is absent in DNA, the polymer is much more stable and lasts for a much longer time than it would with the hydroxyl. Thus, DNA can act as a stable long-term repository for genetic information. How stable? RNA is usually degraded within your cells in 30 minutes. On the other hand, DNA lasts your whole lifetime, and intact DNA thousands or millions of years old may be able to be recovered from frozen mammoth carcasses and mosquitoes trapped in amber. |
||
|
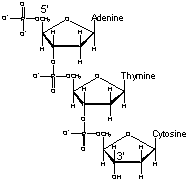
Polynucleotides have a free 5' phosphate group at one end and a free 3' hydroxyl group at the other end. By convention, these sequences are named from 5' to 3'. For example, the molecule shown at right is ATC, not CTA. |
 |
|
Polymerization of Nucleotides