Biomolecules:
Lipids
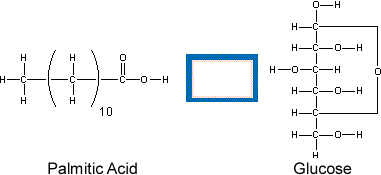
Oxidation State of Fatty AcidsWhy do lipids store so much more energy than carbohydrates? A clue can be found in the oxidation states of the carbon atoms in each molecule. Move your mouse over the structures below to see the oxidation state of each atom. |
||
 |
|
 |
|
Notice how all the carbon atoms in glucose (a sugar) have oxidation states of -1, 0 or +1, while all but one carbon atom in palmitic acid (a fatty acid) have oxidation states of -2 or -3. This means that the carbon atoms in fatty acids have more electrons around them. As described in the Electrochemistry tutorial, when electrons move from an atom with a low affinity for electrons (low electronegativity), like carbon, to one with a high affinity for electrons, like oxygen, energy is released. Therefore, when the greater number of electrons around the carbon atoms in fatty acids are transferred to oxygen (when the fatty acids are oxidized), more energy is released than when the same process happens to carbohydrates. See the Electrochemistry tutorial for more information about oxidation and reduction. |
||
Oxidation States