A Shortcut
Once you are familiar with the method for determining DH outlined on the previous page, think about using this shortcut.
DHº = S [#mol • DHfº(products)] - S [#mol • DHfº(reactants)]
This means that you take each product, multiply its standard enthalpy of formation by the number of moles appearing in the balanced chemical equation, and add them together. Then you do the same for each reactant. Then you subtract the sum for the reactants from the sum for the products, giving you the standard enthalpy change for the reaction.
Why not use this shortcut all the time? Because it is extremely easy to lose track of signs, especially for the reactants. Many of the reactant molecules may have negative DHfº's, which need to be switched to positive when the sum is determined. Still, if you understand what is happening, and are careful, the shortcut will give you the same answer as the method outlined on the previous page.

 Calculate the DHº of photosynthesis, using the shortcut method described here.
Calculate the DHº of photosynthesis, using the shortcut method described here.
6 CO2 (g) + 6 H2O (g) 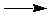 C6H12O6 (s) + 6 O2 (g) C6H12O6 (s) + 6 O2 (g)
|