|
Sometimes enzymes need to be turned off. For example, a complicated system of
enzymes and cells in your blood has the task of forming a clot whenever you
are cut, to prevent death from blood loss. If these cells and enzymes were
active all the time, your blood would clot with no provocation and it would
be unable to deliver oxygen and nutrients to the peripheral tissues in your
body. So enzymes have evolved mechanisms to be turned off, which usually involve inhibitors,
molecules that bind to an enzyme and prevent it from catalyzing its reaction.
There are three general kinds of inhibitors: competitive, noncompetitive, and
mixed inhibitors.
Competitive Inhibitors
In competitive inhibition
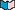 , a molecule similar to the substrate but unable to be acted on by the enzyme competes with the substrate for the active site. Because of the presence of the inhibitor, fewer active sites are available to act on the substrate. But since the enzyme's overall structure is unaffected by the inhibitor, it is still able to catalyze the reaction on substrate molecules that do bind to an active site. Note that since the inhibitor and substrate bind at the same site, competitive inhibition can be overcome simply by raising the substrate concentration.
, a molecule similar to the substrate but unable to be acted on by the enzyme competes with the substrate for the active site. Because of the presence of the inhibitor, fewer active sites are available to act on the substrate. But since the enzyme's overall structure is unaffected by the inhibitor, it is still able to catalyze the reaction on substrate molecules that do bind to an active site. Note that since the inhibitor and substrate bind at the same site, competitive inhibition can be overcome simply by raising the substrate concentration.
At the macroscopic level, the effect of competitive inhibition is to increase
the substrate concentration required to achieve a given reaction velocity;
in other words, to raise the Km. The Vmax is unchanged,
however.
 Select either uninhibited or inhibited from the boxes below. Then click in the image area to see the course of an uninhibited or a competitively inhibited enzymatic reaction.
Select either uninhibited or inhibited from the boxes below. Then click in the image area to see the course of an uninhibited or a competitively inhibited enzymatic reaction.
|
|
 Methanol (CH3OH) is a poison, not because of what it does to the body itself, but because the enzyme alcohol dehydrogenase oxidizes it to formaldehyde, CH2O, which is a potent poison. A treatment of methanol poisoning is to give the patient ethanol, CH3CH2OH. Why is this effective? Type your explanation in the space provided, then click on the Check button.
Methanol (CH3OH) is a poison, not because of what it does to the body itself, but because the enzyme alcohol dehydrogenase oxidizes it to formaldehyde, CH2O, which is a potent poison. A treatment of methanol poisoning is to give the patient ethanol, CH3CH2OH. Why is this effective? Type your explanation in the space provided, then click on the Check button.
Ethanol is a competitive inhibitor of methanol to alcohol dehyrogenase. It competes with methanol for the active site. Thus, as ethanol is added, less methanol can bind to alcohol dehydrogenase's active sites. Formaldehyde is produced at a slower rate, so the patient doesn't get as sick.
|