|
For example, the amino acid alanine noncompetitively inhibits the enzyme pyruvate kinase. Alanine is one product of a series of enzyme-catalyzed reactions, the first step of which is catalyzed by pyruvate kinase.
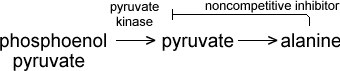
 Why does it make sense for the product of an enzymatic chain of reactions to inhibit one of the enzymes earlier in the chain? Type your answer in the space provided, then click on the Check button.
Why does it make sense for the product of an enzymatic chain of reactions to inhibit one of the enzymes earlier in the chain? Type your answer in the space provided, then click on the Check button.
This prevents the unnecessary buildup of molecules. Once the cell has enough alanine, for example, it uses alanine to shut off the chain that produces more.
Some inhibitors have the effects of both competitive and noncompetitive inhibition, i.e., they affect both the enzyme's affinity for substrate and the maximal rate of catalysis. Such inhibitors are called mixed inhibitors.
|