Coupling Reactions
Chemical reactions with a positive DG have been described here as reactant-favored, meaning that, when the reaction is completed, there are more reactants that products. For example, on previous pages you have calculated that the decomposition of calcium carbonate:
CaCO3(s) 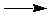 CaO(s) + CO2(g) DGº = 130.40 kJ CaO(s) + CO2(g) DGº = 130.40 kJ
is reactant-favored. Does this mean that this reaction cannot be made to yield products? No. In fact, you have also determined that if the temperature is raised above 837 ºC, this reaction becomes product-favored. How can the temperature be raised enough to cause this? One possibility is to heat the calcium carbonate over a coal fire:
C(s) + O2 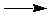 CO2(g) DGº = -394.359 kJ CO2(g) DGº = -394.359 kJ
The heat released by burning the coal raises the temperature enough that the decomposition of calcium carbonate becomes product-favored. If you add the two equations and apply Hess's Law:
CaCO3(s) + C(s) + O2 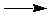 CaO(s) + 2CO2(g) DGº = -263.96 kJ CaO(s) + 2CO2(g) DGº = -263.96 kJ
You see that the overall equation is product-favored. This is an example of coupling  : a reactant-favored reaction is linked to a product-favored reaction so that both reactions yield products. : a reactant-favored reaction is linked to a product-favored reaction so that both reactions yield products.
|