Biomolecules:
Protein 1
Amino Acids
Proteins are polymers of amino acids
![]() .
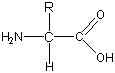
An amino acid is a carbon atom (called the a carbon) bonded to a hydrogen atom, an amine group, a carboxylic acid group, and one of 20 different side chains. The structure of an amino acid is shown at right (R is a generic letter used to take the place of the side chain). All 20 amino acids have three-letter abbreviations for their names. For example, Val is the abbreviation for valine.
.
An amino acid is a carbon atom (called the a carbon) bonded to a hydrogen atom, an amine group, a carboxylic acid group, and one of 20 different side chains. The structure of an amino acid is shown at right (R is a generic letter used to take the place of the side chain). All 20 amino acids have three-letter abbreviations for their names. For example, Val is the abbreviation for valine.
The 20 amino acids are sorted into 4 groups, hydrophobic, polar but uncharged, basic, and acidic, depending on the nature of their side chains (R groups).
On the structure of valine (Val) at the right, try clicking on the following groups:

|
a carbon |
|
The other groups are bonded to the a carbon |
|
Side chain |
|
The side chain is usually represented by an R. |
|
Amine |
|
Amine groups contain nitrogen |
|
Acid |
|
The acid is a carboxylic acid (COOH) |
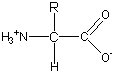 The amine group of an amino acid has a relatively high pKa, so at physiological pH (about 7), it will tend to bind a proton, becoming positively charged. Similarly, the acid group has a relatively low pKa, so around pH 7 it will tend to donate its proton to water, becoming negatively charged. Thus, amino acids usually have both a positive charge on the amine group and a negative charge on the acid group. This form of an amino acid is called the zwitterion form (German for "double ion").
The amine group of an amino acid has a relatively high pKa, so at physiological pH (about 7), it will tend to bind a proton, becoming positively charged. Similarly, the acid group has a relatively low pKa, so around pH 7 it will tend to donate its proton to water, becoming negatively charged. Thus, amino acids usually have both a positive charge on the amine group and a negative charge on the acid group. This form of an amino acid is called the zwitterion form (German for "double ion").
Amino Acids