Planarity of Peptide Bonds
Interestingly, peptide bonds have a second resonance form, as demonstrated below. This means that the peptide bond (the C=O and N-H) all reside in a single plane. Thus, there is no rotation around the 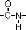 bond. bond.
 Click on the structure below to switch the resonance forms of the peptide bond.
Click on the structure below to switch the resonance forms of the peptide bond.
|