Biomolecules:
Protein 1
Sickle Cell AnemiaHemoglobin (Hb) is a complicated molecule composed of four protein chains and four small non-protein molecules, called heme, that carries oxygen from the lungs to the rest of the body. Hb has two subtly different forms, one when it has bound oxygen and another when the oxygen has dissociated from it.
|
|
|
As discussed in the debriefing, sickle cell anemia is caused by the substitution of a single acidic amino acid, ßGlu6 to Val. As the structure shows, this amino acid position is on the surface of the protein. When the sickle-cell Hb, also called HbS, loses oxygen, another hydrophobic patch becomes exposed in both normal Hb and HbS (this patch is formed by ßPhe85 and ßLeu88) which the new hydrophobic Val is in a position to interact with (see the diagram below). Large fibrous aggregates of Hb form, thousands of Hb molecules in length, that distort the red blood cell into a sickle shape. |
|
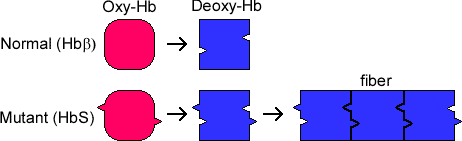 Thus, the symptoms of sickle cell anemia are due to the aggregate of Hb that forms in the red blood cells. Oxygen can't get into the HbS fibers as well, so each red blood cell carries less oxygen than one with normal Hb. Because of the fibers within the cell, the cells are less flexible and tend to clump up and block the tiny capillaries they have to pass through. Tissues suffer damage from the hard red blood cells passing through the confined capillaries and have poor oxygen content, both because each cell carries less oxygen and because normal cells can't get into the tissue. Because oxygen flow is so poor, the patient becomes fatigued more easily. |
|
Return to the Biomolecules gateway page