|
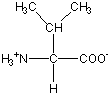
Amino acids are grouped according to what their side chains are like. The nine amino acids that have hydrophobic
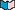 side chains are glycine (Gly), alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile), proline (Pro), phenylalanine (Phe), methionine (Met), and tryptophan (Trp). Shown at the right is the structure of valine. These side chains are composed mostly of carbon and hydrogen, have very small dipole moments, and tend to be repelled from water. This fact has important implications for proteins' tertiary structure (see the Proteins 2 module for a discussion of tertiary structure).
side chains are glycine (Gly), alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile), proline (Pro), phenylalanine (Phe), methionine (Met), and tryptophan (Trp). Shown at the right is the structure of valine. These side chains are composed mostly of carbon and hydrogen, have very small dipole moments, and tend to be repelled from water. This fact has important implications for proteins' tertiary structure (see the Proteins 2 module for a discussion of tertiary structure).
|
 |
|
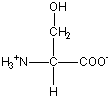
Six amino acids have side chains that are polar
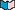 but not charged. These are serine (Ser), threonine (Thr), cysteine (Cys), asparagine (Asn), glutamine (Gln), and tyrosine (Tyr). These amino acids are usually found at the surface of proteins, as discussed in the Proteins 2 module. Shown at the right is the structure of serine.
but not charged. These are serine (Ser), threonine (Thr), cysteine (Cys), asparagine (Asn), glutamine (Gln), and tyrosine (Tyr). These amino acids are usually found at the surface of proteins, as discussed in the Proteins 2 module. Shown at the right is the structure of serine.
|
|